Why Alcohols and glucose are not Acids?
Compounds such as alcohols and glucose contain hydrogen but are not categorized as acids. Describe an activity to prove it.
To understand why alcohol and Glucose compounds are not categorized as acids. Let’s first understand what Acid is?
What is Acid?
An acid is a chemical substance that donates proton or Hydrogen ions or accepts electrons.
In other words, the chemical substance that donates Hydrogen ions (H+) is called an Acid.
The acidity of acids is attributed to the H+ ions
Acid Solution:
Acid solutions have ions and the moment (i.e., movement) of ions in the solution helps for the flow of electric current through the solution.
Activity to prove that alcohols and glucose contain hydrogen but are not categorized as acids
Aim: To prove that Alcohols and Glucose contain Hydrogen but they are not acids.
Materials required: Solutions of Hydrochloric acid, Glucose and Alcohol, 100 ml beaker, 2 different coloured electrical wires, graphite rods, bulb, 230 V AC plug.
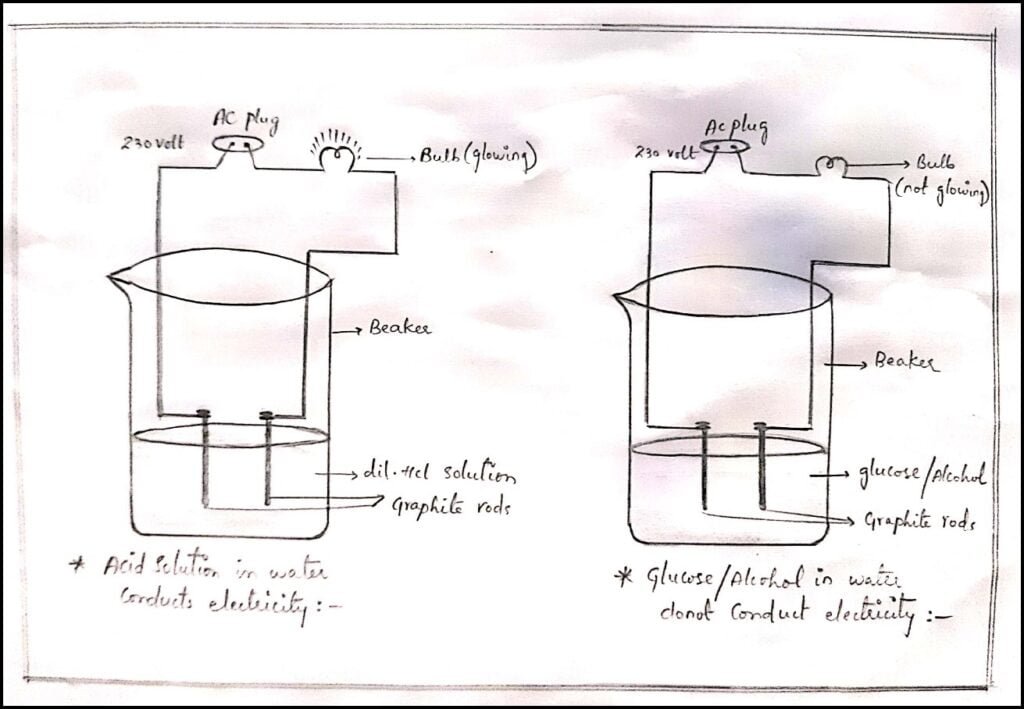
Procedure:
1. Prepare solutions of Hydrochloric Acid, Glucose and Alcohol.
2. Connect 2 different coloured electrical wires to graphite rods separately in a 100 ml beaker as shown in the figure.
3. Connect the free ends of the wire to the 230-volt AC plug and complete the circuit as shown in the figure by connecting a bulb to one of the wires.
4. After completing the circuit, pour dilute HCL in the beaker,
5. Now switch on the current,
6. You will notice that the bulb which is connected in the circuit will start glowing.
7. Now repeat the same experiment with Glucose or Alcohol solution.
8. After switching on the circuit in the Alcohol or Glucose solution, you will notice that the bulb didn’t glow in this case.
9. The glow of the bulb indicates that there is a flow of electric current through the solution.
As discussed above, acid solutions have ions and the moment of the ions in the solution helps for the flow of electric current through the solution.
Conclusion: Through this activity, we conclude that Glucose and Alcohol didn’t produce H+ ions in aqueous solution. Hence, they are not acidic.
Precautions:
1. While diluting acid add concentrated acid into the water slowly while continuously stirring. Don’t add water to the acid, it can cause an explosion and burning of skin.
2. Note that the graphite rods are immersed in water.
