Q: Collect the Information about the scientists who developed atomic theories.
Ancient Greek Beliefs About Atom
Leucippus and Democritus were the first to propose, in the fifth century B.C., that all matter is made of tiny units called atoms. The two philosophers held that these were solid particles without internal structure, and came in a variety of shapes and sizes. Intangible qualities such as taste and colour, according to this theory, were made of atoms. However, Aristotle strongly opposed this idea, and the scientific community failed to pay serious attention to it for centuries.
Dalton’s Atomic Theory
In 1808, English chemist John Dalton further built on the Greek notion of atoms.

Postulates of Dalton’s Atomic theory :
1. Matter consists of indivisible particles called atoms.
2. Atoms are neither created nor destroyed in a chemical reaction. The reorganisation of atoms occurs in chemical reactions.
3. All the atoms of the same element have identical mass and identical chemical properties. Atoms of different elements have different masses and different chemical properties.
4. Compounds are formed when atoms of different elements combine in simple whole-number ratios. That is, a chemical change is the union or separation of atoms as in whole numbers.
5. When atoms of different elements combine in a different whole number of ratios they form different compounds.
e.g.: carbon monoxide (CO); carbon dioxide (CO2).
Hence ‘C’ and ‘O’ combine in 1:1 and 1: 2 ratios respectively to give two different compounds
J.J Thomson’s Model of the Atom
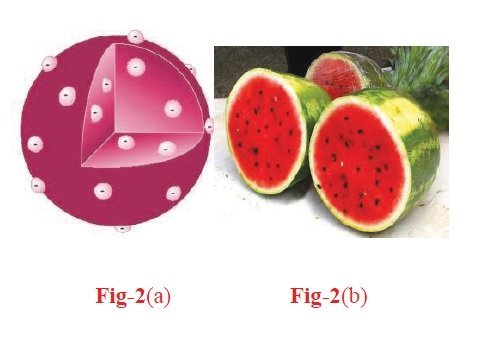
This atomic model was proposed by J.J. Thomson in 1898. This model was commonly called the plum pudding model, referring to the way the fruit pieces are distributed throughout a plum pudding.
According to this model:
1. An atom is considered to be a sphere of uniform positive charge and electrons are embedded in it, as shown in the figure
2. The total mass of the atom is considered to be uniformly distributed throughout the atom.
3. The negative and the positive charges are supposed to balance out and the atom as a whole is electrically neutral.
A more familiar example that represents Thomson’s atomic model is the watermelon (Figure 2 (b)).
The positive charge is spread throughout the atom like the red part of the watermelon. The black seeds distributed throughout the red part represent electrons.
Thomson’s model was modified by one of his students.
Rutherford’s Nuclear model of an atom
Rutherford’s alpha particles scattering experiment
Ernest Rutherford was a scientist born in New Zealand. In 1909, he did some experiments using gold foil and alpha particles. Alpha particles consist of two protons and two neutrons bound together. Since they do not have any electrons, they are positively charged with two units of charge.
Let us see the experimental setup and understand Rutherford’s experiment. There is a source of fast-moving alpha particles which have a considerable amount of energy. The stream of alpha particles is directed towards a very thin gold foil.
The gold foil was placed inside a detector in such a way that the detector would show a flash of light when an alpha particle struck it (see fig-3). The entire arrangement was kept in a vacuum chamber.


It was found that most of the alpha particles passed straight through the atoms without any deflection, and some particles deflected at small angles. Only very few particles were deflected through large angles and a very very small number of particles were reflected right back as shown in the figure.
Features of Nuclear Model of Atom
On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features:
- All the positively charged particles in an atom formed a small dense centre, called the nucleus of the atom. The electrons were not a part of the nucleus.
- He also proposed that the negatively charged electrons revolve around the nucleus in well-defined orbits. Rutherford’s model is sometimes referred to as the planetary model because the motion of the electrons around the nucleus resembles the motion of the planets around the Sun.
- The size of the nucleus is very small as compared to the size of the atom.
Limitations of Rutherford’s atomic model
Think of an atom like hydrogen with one electron and one proton. The electron is attracted by the proton in the nucleus.
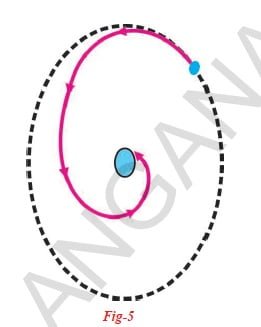
Even in a circular motion around the nucleus, the electron should have an acceleration. An accelerated charged particle moving in a circular path would always radiate energy continuously. Thus, the revolving electron would lose energy continuously and get directed towards the positively charged nucleus as shown in the figure and eventually crash into the nucleus. If this is true, the atoms would become highly unstable and the matter would not exist in the form that we see it now. But we know that atoms are stable.
Bohr’s Model of the Atom
In order to overcome the limitations of Rutherford’s model, in 1913, Niels Bohr put forward a thought that electrons can be found only in certain energy levels, or regions, around the nucleus. Electrons must gain energy to move to a higher energy level or they must lose energy to move to a lower level.
Postulates of Boh’s Atomic Model
Restricting the path of electrons inside the atom, Neils Bohr made the following postulates about the model of an atom:
1. The electrons revolve around the nucleus in certain, discrete circular orbits of the atom. These orbits or shells are called energy levels.
2. While revolving in these discrete orbits the electrons do not radiate energy and this is the reason why electrons do not fall into the nucleus.
3. The electron orbits or shells are represented by the letters K, L, M,N… or the numbers, n=1, 2, 3, 4,….. as shown in the figure.

Niels Bohr could successfully explain the properties of a hydrogen atom like the atomic spectra emitted by a hydrogen atom employing this model but this model could not predict the spectra of atoms or ions with more than one electron.
Until Rutherford’s and Bohr’s time, the neutron had not been discovered. Neutron was discovered nearly two decades later. Except for the hydrogen atom, the atoms of all other elements contain neutrons in their nuclei.
Bohr-Sommerfeld model of an atom
In an attempt to account for the structure (splitting) of line spectra known as fine spectra, Sommerfeld modified Bohr’s atomic model by adding elliptical orbits. While retaining the first of Bohr’s circular orbits as such, he added one elliptical orbit to Bohr’s second orbit, two elliptical orbits to Bohr’s third orbit, etc., such that the nucleus of the atom is one of the principal foci of these elliptical orbits. He was guided by the fact that, in general, periodic motion under the influence of a central force will lead to elliptical orbits with the force situated at one of the foci.
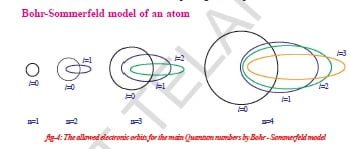
Bohr-Sommerfeld’s model, though successful in accounting for the fine-line structure of hydrogen atomic spectra, does not provide a satisfactory picture of the structure of atoms in general.
This model failed to account for the atomic spectra of atoms of more than one electron.
Quantum mechanical model of an atom
If the electrons are not distributed in orbits around the nucleus, an atom does not have a definite boundary.
As a result, it is not possible to pinpoint an electron in an atom. Under these circumstances in order to understand the properties of electrons in an atom, a quantum mechanical model of an atom was developed by Erwin Schrodinger.
According to this model of an atom, instead of orbits of Bohr’s model, the electrons are thought to exist in a particular region of space around the nucleus at a given instant of time The region of space around the nucleus where the probability of finding the electron is maximum is called an orbital.
Quantum numbers
Each electron in an atom is described by a set of three numbers n, l, and ml .These numbers are called quantum numbers. These numbers indicate the probability of finding the electron in the space around the nucleus.
The quantum numbers describe the space around the nucleus where the electrons are found and also their energies. These are called atomic orbitals.
- Principal Quantum Number (n)
- The angular-momentum quantum number (l)
- The magnetic quantum number (ml)
- Spin Quantum Number (ms)
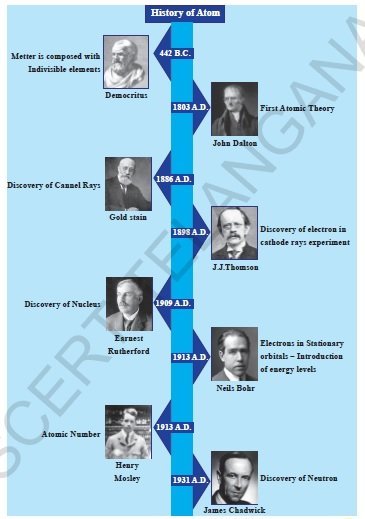
The model of the atom, as we know it today, is the contribution of many scientists. Let us observe the history of Atom in the chart.
Sources: Bajrai Online Solutions completed this project with the help of Telangana State Class IXth and Xth textbooks and starting part from sciencing.
————–End of the Project———–
