What is meant by “Water of crystallization” of a substance? Describe an activity to show the water of crystallization.
Water of Crystallization:
According to the Physics TextBook,
Water of Crystallization is the fixed number of water molecules present in one formula unit of a salt.
A substance containing water of crystallization is called a Hydrous substance or hydrate.
Water of Crystallization can be removed by heating the substance. After removing water from crystallization, the salt is called anhydrous salt.
Formula Unit:
A Formula Unit is defined as the simplest whole number ratio of ions in the ionic bond.
The formula unit of Water is H2O/ The formula unit of Sodium Chloride is NaCl, and CO2 is the formula unit of Carbon dioxide.
For example, let us take Copper Sulphate. The Chemical formula of Copper Sulphate is CuSO4.5H2O.
So, by this, we can say that 5 molecules of Water are present in one formula unit of Copper Sulphate.
The other example is Ferrous Sulphate.
The chemical formula of Ferrous Sulphate is FeSO4.7H2O i.e., Ferrous Sulphate has 7 molecules of water.
A famous example of Water of Crystallization is the Plaster of Paris
The Chemical formula of Plaster of Paris is CaSO4. ½ H2O or we can write it as 2 CaSO4.H2O.
We can say that 2 molecules of Plaster of Paris contain 1 molecule of water in it.
The water of Crystallization is responsible for the colour of the crystal as well as the shape of the crystal.
Let’s understand it with an activity
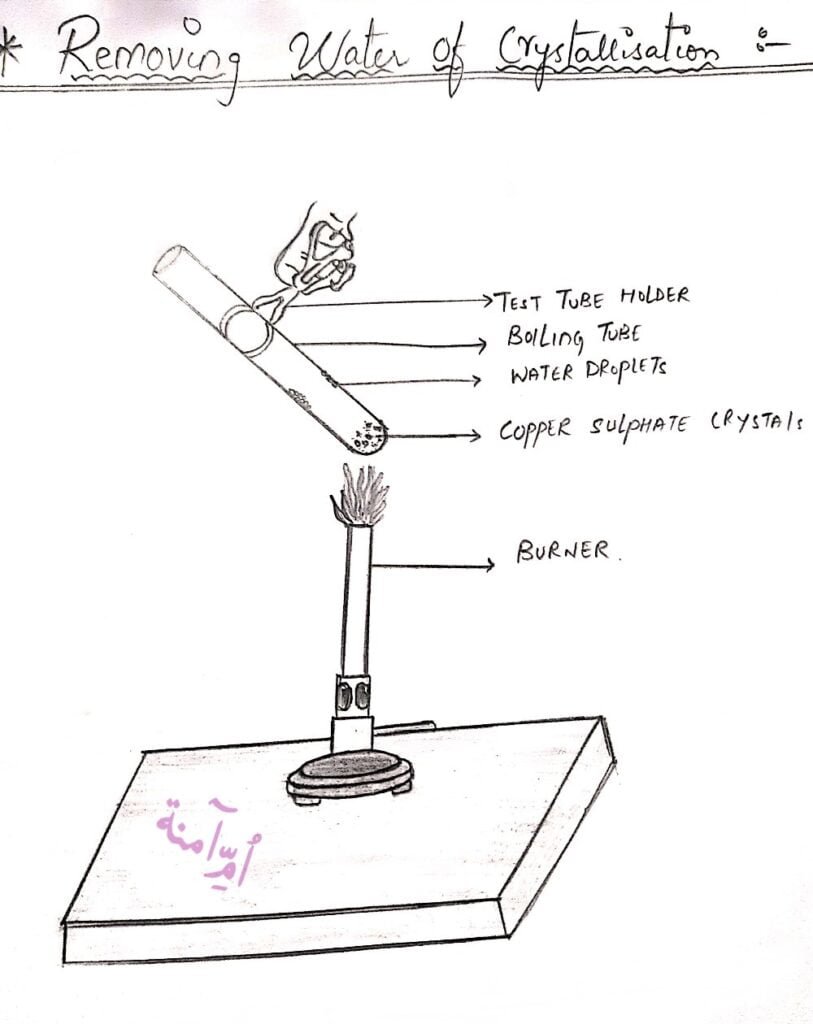
Activity to show the water of crystallization of Copper Sulphate
Aim: To Show that Copper Sulphate has Water of Crystallization
Requirements: Crystals of Copper Sulphate, test tube, Test-tube stand, Burner, water.
Procedure:
1. Take some Crystals of Copper Sulphate and put them in a test tube,
2. Note the Colour of Copper Sulphate crystals. You will notice it is blue.
3. Now heat the test tube with the burner for some time,
4. You will notice water droplets at the corners of the test tube,
5. You will also notice that the copper sulphate which was blue earlier, changes to white.
6. Now pour some water droplets into the white colour copper sulphate powder,
7. You will notice that Copper sulphate regains its BLUE colour and Crystalline form.
Conclusion: Through this activity, we conclude that Crystal salts have Water of Crystallization in them. The Colour and shape of the salts are due to Water of Crystallization.

